Pharmaceutical & Life Sciences - Improve Medication Adherence & Study Compliance
Mean adherence rates in clinical trials for appointment keeping, short and long-term medication taking and appointment keeping range from 39% – 63% for prevention trials and 59% – 78% for treatment trials.1 Nonadherence in research settings occurs as participants deviate from treatment protocol e.g. forgetting to take doses, taking medications at incorrect times, taking an incorrect dose or missing study visits. Nonadherence by participants can lead to underestimated harms, misinterpretation of results, type II error and low statistical power – all leading to increased costs.
A meta-analysis of trials of interventions to improve medication adherence showed increases in adherence in the range of 4% – 11%.2 This was achieved through two types of interventions: behavioural and educational.
- Behavioural Interventions included the use of any tool or action that would change a participant’s skill level or normal routine, including reminders, and dosage schedule changes.
- Educational interventions were those that taught the participant about the medication and how to take it through oral, written, or audiovisual communication.
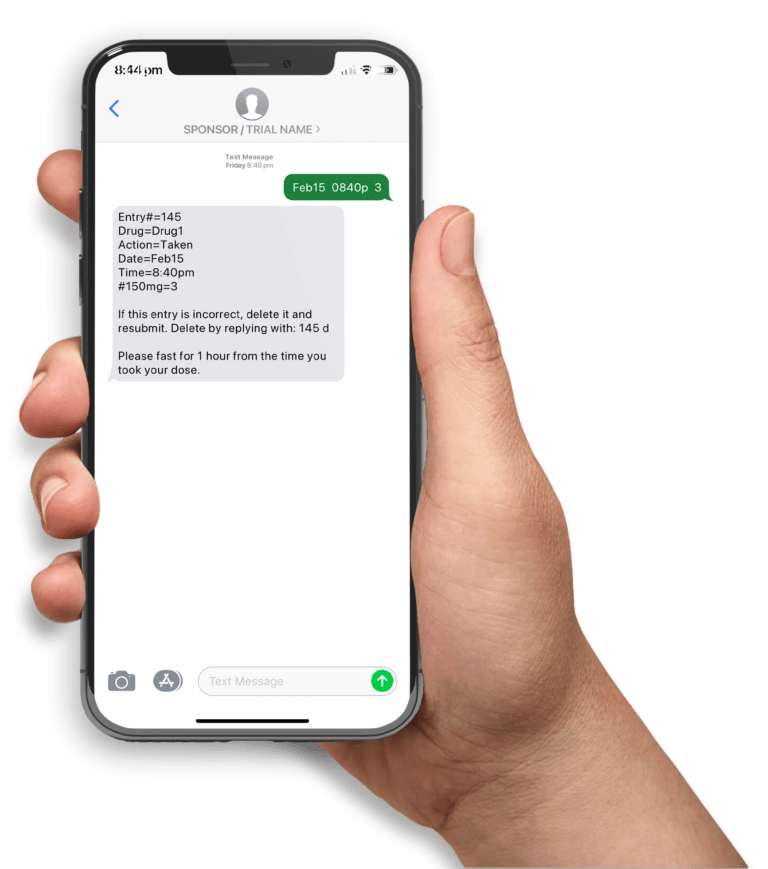
A mobile clinical trial app helps participants follow a medication regimen and subsequently improve study compliance. High adherence rates can be achieved by sending participants timely messages including appointment, medication and diary entry reminders and motivation messages. Moreover, diary entry submissions and other forms of data collection can be automated with the use of mobile phones. Given the ubiquitousness of text messaging (ie. available on ALL mobile phones), this is a popular technology platform to support clinical trials.
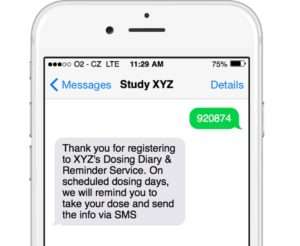
Enrol
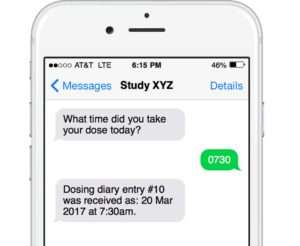
Engage
Manage
Reminders and appointment schedules can be managed online. Escalations are logged as they arise and are flagged until they are addressed. An audit trail is maintained as Study Team members add, edit and delete data.
Analyse
The Study Team can review, analyze and extract all participant data via the web portal. Insights into study performance can be provided across various levels including participant, site and geography.
Clinical Trial App is driven by Synegys' mComply
The clinical trial app ensures medication adherence and delivers overall study compliance. It is built on Synegys’ mComply platform and is customisable to suit any study’s needs. The solution can be used with text messaging on feature phones or as a mobile health app on smartphones / tablets across different operating systems (iOS, Android). The former is the most popular variant given its ubiquitous nature and lower cost to implement for most study trials
Check out our clinical trial app video to see how Synegys’ mComply supports diary entry submissions and reminders.
mComply Business case for mobile Clinical Trial Automation
Synegys’ mComply solution helps studies achieve the level of participant compliance and retention they need to stay on track for success. The solution focuses on text messaging interventions, a ubiquitous approach to realise adherence rates in excess of 97%.
Consider a scenario of running a Phase 2 study with 203 participants at 30% noncompliance. Each participant is to follow a medication regimen every 3 days for 3.5 months with study appointments every month. Reducing nonadherence to 3% to maintain equivalent statistical power results in nearly $12 million in cost of capital savings. When only considering variable operating expenditures, $1.5 million can be saved through lower enrolment and retention costs, reduced compliant episodes, improved data collection and reduced “no-shows”.
mobileHealthWorks
Our mobileHealthWorks division provides more information and examples of CFR-compliant and HIPAA-ready apps to deliver medication text message reminders, appointment scheduling, wellness surveys, and dosing entries in clinical trials.
1 Robiner, William N. Enhancing adherence in clinical trials. Contemporary Clinical Trials. 2005;26:59–77.
2 Andrew M. Peterson, Liza Takiya, Rebecca Finley. Meta-Analysis of Trials of Interventions to Im- prove Medication Adherence. Am J Health Syst Pharm. 2003;60(7).
3 Eastern Research Group, Inc. Examination of Clinical Trial Costs and Barriers for Drug Develop- ment. July 25, 2014.
4 Wittes, Janet. Sample Size Calculations for Randomized Controlled Trials. Epidemiologic Reviews. Vol 24, No. 1, 2002.
5 Damodaran, Weighted Average Cost of Capital for drugs (biotechnology). http://people.stern.nyu. edu/adamodar/New_Home_Page/data.html. Updated Jan 5, 2018.
6 CMR International. Pharmaceutical R&D Factbook 2011. Thomson Reuters, May 2011.
7 DiMasi, Briefing – Cost of Developing a New Drug. Nov 18, 2014. https://csdd.tufts.edu/cs-ddnews/2018/3/9/march-2016-tufts-csdd-rd-cost-study.
8 Biotechnology Innovation Organization. Clinical Development Success Rates 2006-2016. 2016.

