Over twenty years ago, Dr. John Urquhart published a paper titled, “Patient Non-Compliance with Drug Regimens: Measurement, Clinical Correlates, Economic Impact.”[1] Urquhart argued that when patients skip doses, take medications off schedule or don’t adhere to trial requirements, Sponsors can arrive at erroneous conclusions. Since his publication, new trial designs and methods of statistical analysis have indirectly addressed the issue. However, medication nonadherence and trial noncompliance continues to persist, contributing to rising clinical trial costs.
To illustrate the financial impact of medication nonadherence, we consider a realistic scenario of a Phase II trial having an industry average of 203 enrolled patients.[2] The trial has a constant investigational medicinal product (IMP) nonadherence rate of 30%. We review the impact of nonadherence on the study’s power and sample size, cost of capital and opportunity costs, recruitment and retention costs, noncompliance episodes and missed appointments. We then share how compliance tools like Synegys’ mComply can improve a study’s financial performance.
Power & Sample Size
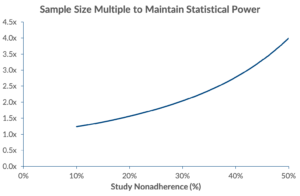 With studies averaging trial adherence rates ranging between 59% – 78%[3], statistical power and sample size become a concern. As adherence rates drop, required sample size increases exponentially. To account for noncompliance, Wittes states that sample size needs to be inflated by the square of the proportion of people in both treated and control groups who deviate from study protocol.[4] In our scenario, sponsors need to enrol an additional 104 patients (totalling 203 patients[5]) in order to maintain equivalent statistical power.
With studies averaging trial adherence rates ranging between 59% – 78%[3], statistical power and sample size become a concern. As adherence rates drop, required sample size increases exponentially. To account for noncompliance, Wittes states that sample size needs to be inflated by the square of the proportion of people in both treated and control groups who deviate from study protocol.[4] In our scenario, sponsors need to enrol an additional 104 patients (totalling 203 patients[5]) in order to maintain equivalent statistical power.
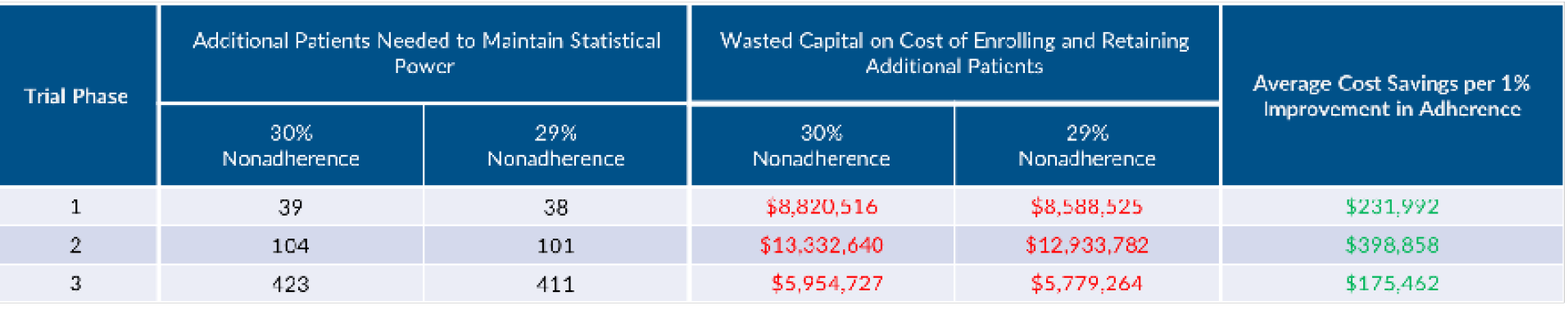
As studies enrol more patients, operating costs associated with recruitment and retention rises accordingly. In our scenario where we need to enrol an additional 104 patients to maintain equivalent statistical power, nearly $1.7 million in direct variable costs will be incurred (patient recruitment and retention, registered nurse/CRA, physician, clinical procedure and central lab costs).[6]
Cost of Capital / Opportunity Costs
Alsumidaie[7] examined how nonadherence influences clinical trial duration and cost. Expanding on Alsumidaie’s work, we calculate the estimated wasted capital on cost of enrolling and retaining 104 patients in our study scenario to be $13.3 million. By reducing non-adherence by 1% (to 29%), sponsors need to enrol 3 less patients in order to maintain equivalent statistical power, resulting in nearly $399,000 in cost savings, and minimising timeline slippage.[8],[9],[10]
Note that our analysis differs from Alsumidaie’s as we use a different IMP nonadherence rate, include the aforementioned direct variable cost categories, employ a weighted average cost of capital (WACC) of 9.24%, apply a 19.9% success rate from Phase II to approval and inflate the sample size using Witte’s model rather than use an imputed linear sample size multiple as he does.
Wasted Retention and Recruitment Costs
Wasted retention and recruitment costs are incurred with each patient drop out. With 18% of screened patients dropping out[11], $591,600 of direct costs are wasted.
Dealing with Noncompliance Episodes
There is an immediate cost when dealing with noncompliance episodes. Assuming patients need to follow a 3-day dosing regimen over a 3.5 month period and the cost for dealing with an episode of noncompliance is $102[12], $218,300 is spent dealing with noncompliance episodes.
Missed Study Appointments
Patients failing to attend their study appointments will also raise costs. In fact, mean adherence rates in clinical trials for appointment keeping was found to be 39% and 78% for prevention and treatment trials respectively.[13] In our scenario we consider each patient to have a study appointment scheduled every month for 4 months. With a 30% nonadherence rate, the cost of “no-shows” is estimated to be approximately $40,200.[14]
Improve Medication nonadherence with Patient Engagement Software – mComply
Although noncompliance has been an industry burden for decades, the issue can be tackled by employing cost-effective tools. Armed with a patient engagement tool such as Synegys’ mComply, study teams can manage behaviours that impact statistical power and sample size, ensure patients are following the drug regimen of taking the correct dosage on-schedule, and ensure study timelines are met. mComply engages patients through one or two-way communications, timely notifications and data collection methods accessible on all mobile platforms and phones.
Monitor, Analyze and Act
Self-reported paper diary is a traditional way for pharmaceutical companies to monitor medication utilization. Unfortunately, this is prone to recall bias and does not remind patients to follow a specific medication regimen. By employing electronic patient diaries ranging in complexity from mobile apps to 2-way text messaging, data is time-stamped and captured in near real-time. Electronic-based methods can save trial sponsors nearly 30% in data collection costs.
Using mComply, researchers can access real time data to assess compliance. Automated algorithms can detect symptom changes to proactively detect clinically significant events to support power. mComply also enables correction of nonadherence by alerting trial managers who to counsel and/or remove “professional” or “trouble” patients. By monitoring activities in real time, trial managers can act immediately on power-reducing behaviours so time is not wasted in collecting poor data.
Achieve Adherence Rates Exceeding 97.5%
Some trials consider adherence rates of greater than 80 percent to be acceptable, whereas others consider rates of greater than 95 percent to be mandatory for adequate adherence.[15] Patients regularly achieve high compliance rates of more than 97.5% with mComply. Moreover, they are comforted by the notion that someone is watching over them.
Reduce Patient Dropout
By focusing on reducing study noncompliance, less effort needs to be spent on patient recruitment. Studies employing mComply have seen patient drop out decline by as much as 50%. This is significant as according to the New England Journal of Medicine, it costs an average of $6,533 to recruit a patient for a trial, and three times that amount to recruit a new patient if one is lost due to noncompliance.
Improve Trial Timelines & Save on Opportunity Costs
Clinical trials typically last 42 percent longer than expected in Phase I, 31 percent longer in Phase II, and 30 percent beyond planned deadlines in Phase III – all because of recruitment delays.[16]Assuming a 97.5% adherence rate with mComply, 12 additional patients (as opposed to 104 at a 70% adherence rate) need to be enrolled to maintain equivalent statistical power. Nearly $1.5 million in variable costs can be avoided, translating to $11.8 million in expected cost of capital savings.
Medication Adherence using Mobile Health App or Text Messaging Service
A 2014 McKinsey survey found that “starting small” and “acting fast” is what patients really want when it comes to digital experiences. Highly innovative, fancy apps and wearables were found to be less important to patients than simple, straightforward mobile health tools. The advice, therefore, is to go for “quick wins”[17] that show ROI – in healthcare outcomes, cost savings and revenue.
Synegys’ patient adherence software provides appointment and medication reminders, data collection and more. The patient adherence software immensely improves a clinical trial’s financial profile. The solution comes in two variants – secure text messaging solution and mobile app. The 2-way messaging platform supports data collection and promotes better adherence through reminders and/or notifications. Moreover, mComply’s text messaging variant can be deployed anywhere in the world. For added functionality, a mobile app version is also available to keep clinical trial participants adherent. In addition to sending participants reminders and notifications about following a medication regimen, the mobile app includes electronic informed consent documents and information about upcoming clinical visits. Moreover, the video function on smartphones and tablets can be utilized to conduct clinical visits remotely. Both solutions are customizable, cost-effective, validated and conform to local regulatory and privacy laws.
To learn more, request our brochure to improve medication adherence in clinical trials published by our healthcare division – mobileHealthWorks.
References
[1] J. Urquhart.Patient non-compliance with drug regimens: measurement, clinical correlates, economic impact. European Heart Journal 1996; 17 (Suppl A): 8-15.
[2] Source of information used in this analysis for aggregated clinical trials and patient average data: www.clinicaltrials.gov via www.karmadata.com
[3] Robiner, William N. Enhancing adherence in clinical trials. Contemporary Clinical Trials. 2005;26:59–77.
[4] Wittes, Janet. Sample Size Calculations for Randomized Controlled Trials. Epidemiologic Reviews. Vol 24, No. 1, 2002.
[5] Source of information used in this analysis for aggregated clinical trials and patient averagedata: www.clinicaltrials.gov via www.karmadata.com
[6] Eastern Research Group under contract to the US Department of Health and Human Services. Examination of Clinical Trial Costs and Barriers for Drug Development. July 2014.
[7] Alsumidaie. Non-Adherence: A Direct Influence on Clinical Trial Duration and Cost. Applied Clinical Trials. Apr 2017. http://www.appliedclinicaltrialsonline.com/non-adherence-direct-influence-clinical-trial-duration-and-cost
[8] Damodaran, Weighted Average Cost of Capital for drugs (biotechnology). http://people.stern.nyu.edu/adamodar/New_Home_Page/data.html. Updated Jan 5, 2018.
[9] CMR International. Pharmaceutical R&D Factbook 2011. Thomson Reuters, May 2011.
[10] DiMasi, Briefing – Cost of Developing a New Drug. Nov 18, 2014. https://csdd.tufts.edu/csddnews/2018/3/9/march-2016-tufts-csdd-rd-cost-study.
[11] Forte Research
[12] Only considers the time of a clinical trial manager / coordinator at a 2015 cost of AUD 136.17 (i.e. excludes time from principal investigator and other support departments). Health Consult Pty Ltd. Development of a table of standard costs for conducting Clinical Trials in Australia 2015.
[13] Robiner, William N.: Enhancing adherence in clinical research. Contemporary Clinical Trials 26 (2005). 59-77.
[14] £120 cost for each missed appointment. Department of Primary Care and Social Medicine, Imperial College London, London, UK
[15] Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497
[16] Cutting Edge Information, “Accelerating Clinical Trials: Budgets, Patient Recruitment, and Productivity,” May 2004
[17] McKinsey Healthcare’s Digital Future. Jul 2014.


